On this page
- Summary
-
The disease
-
Routine preventative activities
-
Human disease surveillance objectives
-
Data management
-
Communications
-
Case definition
-
Laboratory testing
-
Case management
-
Environmental risk management
-
Contact management
-
Special situations
-
References
-
Appendices
-
Jurisdiction specific issues
1. Summary
Public health priority
High
PHU response time
NSW specific advice
Urgent for cases currently in dengue-receptive areas and/or cases who have been in dengue receptive areas whilst viraemic, as there is potential for onward local transmission. Routine within 48 hours for cases in areas not receptive to dengue transmission. Enter confirmed cases and probable cases on NCIMS within one working day. See Figure 1 for receptive areas.
Case management
No specific treatment is available for dengue fever. Most cases are self-limiting, with oral fluids and analgesia given acutely. Dengue patients in receptive areas should be advised to avoid being bitten by mosquitoes until fever subsides.
Contact management
In receptive areas only, contact tracing in the form of active case finding of persons in the same exposure area as the case, and recommend laboratory testing of any symptomatic persons.
Note:
For the purposes of this document, receptive areas (darker shading in map below) for dengue are those residential parts (urban, regional or rural) of a defined geographical region in Australia where:
- Aedes aegypti or Aedes albopictus mosquitoes are thought to be present AND
- local transmission has occurred in the past 20 years, OR local public health and entomology authorities consider there to be a risk of local transmission.

Figure 1. The distribution of Aedes aegypti and dengue activity in Queensland* (Source: Queensland Dengue Management Plan 2015-2020).
Note:
- Only urban or residential environments within the shaded areas are potential receptive areas
- Data on vector distribution are patchy and subject to change
2. The disease
Infectious agents
Dengue virus is a flavivirus. Four serotypes of dengue viruses have been described - dengue 1, 2, 3 and 4. Each of the 4 serotypes is capable of causing the full spectrum of clinical manifestations following dengue infection. A fifth serotype was reported to have been identified in Malaysia in 2013; however the human impact of this serotype has not yet been determined and a full description is yet to be published.
Reservoir
Humans; non-human primates such as monkeys maintain the virus in limited forest settings of Asia and Africa.
Mode of transmission
There is no direct person to person transmission of dengue, but transfusion related cases can occur. Transmission is via the bite of an infective female mosquito, principally Aedes aegypti. Aedes aegypti is a highly domesticated urban mosquito found in the tropics and subtropics. In Australia its geographical distribution is currently confined to parts of Queensland. Larvae develop in artificial water-holding containers close to or inside people's homes (such as buckets, tyres, pot-plant bases, roof gutters, rainwater tanks). Aedes aegypti is a day-biting species, with increased biting activity for 2 hours after sunrise and several hours before sunset. Humans are the preferred source of blood meals for Aedes aegypti.
Aedes albopictus can also transmit dengue and is an aggressive day-biting species that also lives around people's homes. It is found in some temperate regions as well as the tropics and subtropics, and in Australia it is currently confined to the Torres Strait islands but could potentially colonise large areas of the mainland. Aedes albopictus breeds in artificial containers and some naturally occurring sites such as tree holes and coconut shells. Adults prefer to rest in heavily shaded outdoor sites; and the female takes blood from a range of mammals.
Vertical transmission from mother to child in utero has been described but is thought to be uncommon and would not be expected in Australia given the current level of dengue activity here. Vertical transmission from infected mosquitoes to their offspring (eggs) has been reported to occur but only at low frequencies. The ecological importance of this phenomenon to dengue persistence is not clear.
Incubation period
The illness typically starts from 4 to 7 days after a person is bitten by an infected mosquito, but ranges from 3-14 days.1 The extrinsic incubation period (EIP) (the incubation period in the mosquito) is from 8-12 days, although shorter EIPs (as low as 5 days) have been reported, leading to explosive outbreaks.2
Infectious period
There is no direct person-to-person transmission of dengue (apart from through blood transfusion). A person with dengue can transmit the virus to mosquitoes from shortly before the onset of symptoms (and febrile period) to the cessation of symptoms: usually 3-5 days. However, to reliably trace possible infectiousness to local vectors, a longer duration of viraemia is assumed, from one day before until 12 days after the onset of symptoms in the case. An infected mosquito can transmit dengue until it dies.
Clinical presentation and outcome
Infection with dengue can produce a wide clinical spectrum of disease, ranging from a mild febrile illness through to a severe, even fatal condition such as dengue haemorrhagic fever (DHF) or severe dengue. The clinical syndrome experienced can be influenced by both age and immunological status.1, 3 Most children infected with dengue experience a mild, undifferentiated febrile illness or asymptomatic infection. For those who develop recognisable signs and symptoms, the clinical course and severity of the disease can be difficult to predict early in dengue infection.1, 3 Typical symptoms of classical dengue include the sudden onset of fever (up to 40°C) accompanied by headache, retro-orbital pain, muscle pains in back and limbs, and rash (erythematous, maculopapular or petechial). Other symptoms include lethargy, weakness, depression, anorexia, taste aberrations (e.g., an unpleasant metallic taste), sore throat, cough, vomiting, abdominal pain and possibly minor haemorrhagic manifestations such as epistaxis, menorrhagia, haematuria and gingival bleeding. Hospitalisation may be required depending on signs of severity such as dehydration, bleeding or comorbidities. Hepatitis is a frequent complication. DHF and dengue shock syndrome (DSS) manifest generally as plasma leakage leading to shock and can be fatal and occur more frequently among children and young adults.
There is no specific treatment for dengue, and care is largely supportive. Oral rehydration and analgesia are routinely used. Intravenous rehydration is the therapy of choice for severe cases, and can ensure that the case fatality rate remains below 1% for these severe cases.4 While the clinical course for dengue is difficult to predict, revised clinical criteria developed by the World Health Organization released in 2009 can aid in the decision making process.
The revised clinical criteria were tested and used in endemic countries (with limited laboratory facilities), to classify dengue cases.4 See Figure 2.
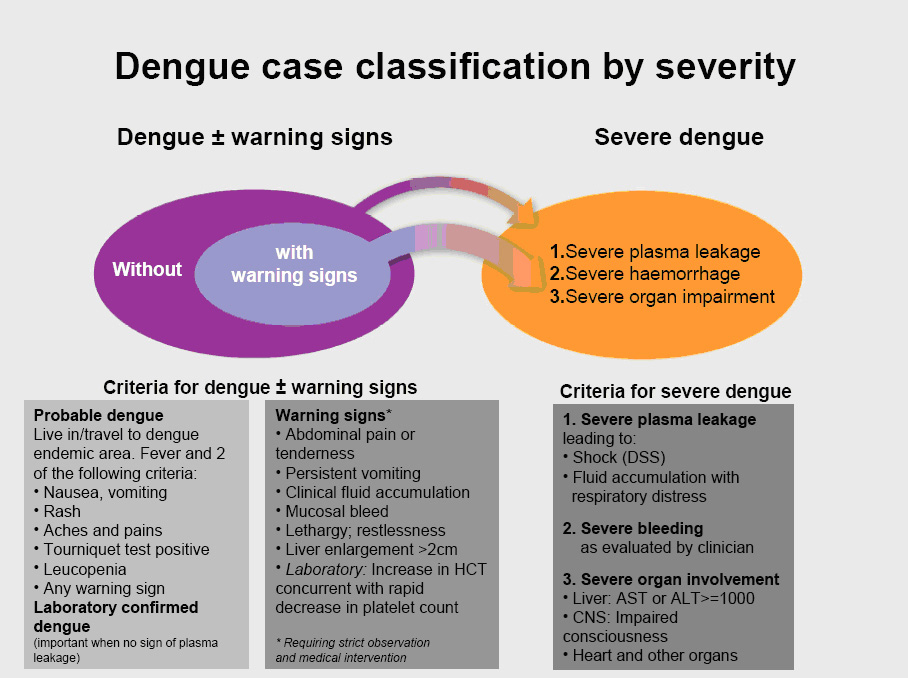
Figure 2. The revised WHO dengue case classification.
Persons at increased risk of disease
Susceptibility to primary dengue infection appears universal. Recovery from infection with one dengue serotype provides lifelong immunity against that serotype but only short-term protection against other serotypes.1 There is increased risk of DHF in secondary dengue virus infections with a different serotype to the primary infection which is thought to be due to differences in immune responses between primary and secondary dengue virus infections.1
Disease occurrence and public health significance
There has been a global resurgence of dengue in the last three decades, with an estimated annual average of 96 million clinical cases occurring in recent years.5 Approximately 2.5 billion people live in areas at risk for epidemic transmission of dengue, most of these in countries of South East Asia and central and South America.5 The global burden of DHF has been estimated at hundreds of thousands of cases each year with case-fatality rates between 1-20% depending on access to effective management.6 Most fatal cases are children and young adults.
In Australia, cases of locally acquired dengue (those acquired in Australia) and Aedes aegypti mosquitoes have historically been reported from most mainland Australian states,7 but recently transmission has been limited to north Queensland. The potential for transmission of the virus is limited by the distribution of the principal vector, Aedes aegypti in urban areas of north Queensland, and Aedes albopictus on some islands of the Torres Strait. There are also increasing number of viraemic travellers arriving into north Queensland. However, a risk of dengue transmission also exists in other parts of central and southern Queensland where Aedes aegypti is currently found, but which are not densely populated by people. In these areas, transmission risk may increase if there is an increase in human population size and where viraemic travellers are present, which may particularly occur in areas with a significant and regular influx of overseas workers such as mining communities. Since 1990, dengue activity initiated by imported cases has increased in north Queensland, with more than 40 discrete outbreaks involving all 4 serotypes and a range of 1-900 reported cases per outbreak.8 In 2008-09 more than 1000 cases of locally acquired dengue encompassing all four serotypes were notified in north Queensland.9 Deaths from DHF occurred in both 2004 and 2008-09 outbreaks after an absence of fatalities for over a century.10 There is a risk dengue could become endemic within Australia, particularly in Cairns. This would increase the likelihood of cases being exported into other towns in the same region and complicate efforts to control the disease.
Notifications of overseas acquired dengue cases have increased over the past few years. The number and proportion of overseas acquired dengue cases in Australia in 2010 and 2011 increased by 298% and 155% respectively. More than half of these cases were acquired in Indonesia, particularly Bali.11
NSW specific advice
The World Health Organization (WHO) Dengue Disease Outbreak News describes the extent of dengue across the Africa, Americas, Eastern Mediterranean, European, South-East Asia and Western Pacific regions. The Western Pacific region reported over 500,000 dengue cases between 1 January 2023 to 7 December 2023.
The extent of dengue transmission activity overseas may be a determinant of risk of local transmission in Australia. Overseas acquired dengue is reported annually from most Australian jurisdictions, but there is only a risk of local transmission when overseas-acquired cases reside for a period in a dengue-receptive region in Australia. In recent decades, the Australian dengue-receptive region comprises settlements of north Queensland (Fig. 1). However, the range of the dengue vector Aedes aegypti extends further south than this, and thus represents some small but finite risk of transmission in certain circumstances.
3. Routine prevention activities
Current prevention activities for dengue depend on reducing human exposure and mosquito control measures. There is no vaccine registered for use in Australia, although a candidate vaccine (developed by Sanofi-Pasteur) is currently in Phase III trials.
NSW specific advice
As of February 2024, no vaccine is routinely available in Australia to prevent dengue infection. In July 2019, the Australian Technical Advisory Group on Immunisation (ATAGI) released clinical advice on the use of Dengvaxia®, a vaccine developed by Sanofi Pasteur, for use on a case-by-case basis. Other dengue vaccines are in varying stages of trials and approvals, however are not yet available in Australia.
Travel advice
Travellers to endemic countries should be advised to take precautions to prevent dengue including:
- ensuring hotel (or any other accommodation) rooms are free of mosquitoes by closing window screens, using insecticide sprays indoors, using bed nets if no window screens
- wearing light coloured, long sleeved clothing in urban or residential areas to minimise skin exposure to day-biting mosquitoes
- wearing permethrin impregnated fabrics
- using an appropriate mosquito repellent containing DEET or picaridin on all exposed skin, and applying frequently and thoroughly according to the manufacturer’s recommendations
- seeking medical advice, as soon as practicable, if they become unwell with a high fever during or soon after travel.
Information on dengue endemic countries can be found on the
HealthMap website.
Mosquito surveillance and control measures
Dengue control aims to break the cycle of transmission through detecting and reducing vector populations. In North Queensland towns with established
Ae. aegypti populations, year-round work is done to educate and support the population to remove domestic container habitats. Mosquito surveillance is required to monitor the presence and numbers of Ae. aegypti (and
Ae. albopictus) in risk areas, ports and airports. These activities require a joint effort from local government, quarantine officers from the Australian Government Department of Agriculture, health authorities and the public. Mosquito killing traps (e.g. lethal ovitraps) and sprays may be added in high risk spots and schools.
For any dengue cases occurring outside north Queensland and not related to exposures overseas, it is imperative that the identity of the vector is determined promptly. This will consist of collection of larvae from flooded artificial containers in yards within 100-200 metres of the case, and collection of adult mosquitoes using traps such as the Biogents Sentinel (BGS) trap or the Gravid Aedes Trap (GAT) within 50 metres of the case’s place of residence. The presence of
Ae. aegypti and
Ae. albopictus is suggestive of dengue transmission risk.
Most outbreaks occur during the summer wet season when vector numbers increase; however, in recent years outbreaks have continued throughout the autumn and winter months.
In dengue receptive areas, an outbreak prompts urgent and intensive mosquito control activities around the addresses where the cases may have been viraemic. This aims to kill infected adult females within a radius of about 200 metres – the distance a vector might fly.8 The primary vector of dengue, Ae. aegypti, is highly domestic and blood-feeds and harbours within premises. Thus, where Ae. aegypti is present, control measures include indoor residual spraying using synthetic pyrethroid insecticides such as bifenthrin and deltamethrin. Outdoor truck mounted ultra-low volume (ULV) or thermal foggers are not effective against Ae. aegypti hidden indoors. Water filled containers are treated with insect growth regulators (e.g. methoprene), Bti or pesticide sprays. Tipping out or removal of containers (source reduction) is effective but laborious and not efficient during large outbreaks. The response when an outbreak occurs is outlined in Section 12 and documented in the
Queensland Dengue Management Plan8.
If the exotic
Ae. albopictus is thought to be the vector, outdoor ULV and harbourage/barrier sprays using residual pyrethroids are effective. Larvae should be controlled as for
Ae. aegypti.
4. Human disease surveillance objective
- To detect and respond promptly to imported cases of dengue into receptive areas.
- To detect and respond promptly to locally acquired cases of dengue in Australia.
- To monitor the epidemiology of dengue to inform development of better prevention and control strategies.
5. Data management
NSW specific advice
Both confirmed and probable cases should be reported to the local Public Health Unit and should be entered in NCIMS within one working day.
When entering potential exposures on NCIMS, the following variables are considered minimum data requirements:
- Place of exposure - enter data in both Clinical and Risk History packages in NCIMS
In the event of an outbreak or enhanced public health investigation, additional data points may be required.
6. Communications
NSW specific advice
For confirmed and probable cases in receptive areas (Figure 1), report the case (on same day of notification) to One Health Branch including case details, onset date and geographical areas of exposure.
For cases acquired in north Queensland or other receptive areas in Queensland that are notified elsewhere, One Health Branch will notify the Communicable Disease Unit in Brisbane (phone: 07 3328 9724 fax: 07 3328 9782) as soon as possible.
For cases imported from overseas and diagnosed in non-receptive areas, routine data entry is sufficient. Entry into NCIMS should occur within one working day and details about the place of exposure/acquisition included under both the 'Clinical' and 'Risk History' packages.
7. Case definition
Both confirmed and probable cases are notifiable. The full surveillance case definition is set out below. Current surveillance case definitions can also be found on the
Department of Health website.
Reporting
Both
confirmed cases and probable cases should be notified.
Confirmed case
A confirmed case requires laboratory definitive evidence
and clinical evidence.
Laboratory definitive evidence*
- Isolation of dengue virus or
- Detection of dengue virus by nucleic acid testing or
- Detection of non-structural protein 1 (NS1) antigen in blood by EIA or
- IgG seroconversion or a significant increase in antibody level or a fourfold or greater rise in titre to dengue virus, proven by neutralisation or another specific test or
- Detection of dengue virus-specific IgM in cerebrospinal fluid, in the absence of IgM to Murray Valley encephalitis, West Nile virus /Kunjin, or Japanese encephalitis viruses
Clinical evidence
A clinically compatible illness (e.g. fever, headache, arthralgia, myalgia, rash, nausea, and vomiting).
Probable case
A probable case requires:
- laboratory suggestive evidence
and clinical evidence
and epidemiological evidence, or
- clinical evidence and household epidemiological evidence
Laboratory suggestive evidence
- Detection of NS1 antigen in blood by a rapid antigen test#,
or
- detection of dengue virus-specific IgM in blood.
Clinical evidence
As for confirmed case.
Epidemiological evidence
- Exposure, between 3 and 14 days prior to onset, in
either
- a country with known dengue activity,
or
-
a dengue-receptive area^ in Australia where a locally-acquired or imported case has been documented with onset within a month.
Household epidemiological evidence
-
Living in the same house ~ as a locally-acquired case in a dengue-receptive area3 of Australia within a month of the onset in the case,
and
- at least one case in the chain of epidemiologically linked cases (which may involve many cases) is laboratory confirmed.
* Confirmation of the laboratory result by an arbovirus reference laboratory is required if the infection was acquired in Australia but outside a dengue-receptive area as defined in the Dengue National Guideline for Public Health Units.# Unless dengue NS1 antigen by EIA is negative
^ As defined in the Dengue CDNA National Guideline for Public Health Units.
~ The case must have spent all the exposure period (from 14 days prior to onset to 3 days prior to onset) living in the same house as the epi-linked confirmed case.
8. Laboratory testing
Testing for dengue is recommended in persons who have a clinically compatible illness and have travelled to an area with known dengue activity during the exposure period (3-14 days prior to onset of symptoms). The suitability of laboratory tests for dengue depends on the timing of a blood sample collected during the illness (see
Figure 3). The timing of laboratory tests for dengue is outlined in
Table 1.

Figure 3: Typical primary dengue infection with timing of diagnostic tests [Source: Tropical Regional Services, Queensland Health]
Table 1. Laboratory tests for dengue
| Up to day 9 from onset of symptoms | PCR and/or NS1 (non-structural protein 1)
and serology | PCR and NS1 are likely to be negative after 7 days, but detections are possible for longer. Serology is likely to be negative in the first 5 days, but it is often helpful to have the earlier blood to show seroconversion |
|---|
| From day 10 onwards | Serology | Serology may have to be repeated |
|---|
* Adapted from Appendix 1: Specific dengue tests, Queensland Dengue Management Plan, 2010-2015
8
The pattern of virus, antigen and antibody production in secondary dengue infections (subsequent infection with a different serotype) is different to primary infections. In secondary infections the predominant immunoglobulin is IgG, and IgM levels are lower than in primary infections. Both antigen detection and PCR are less sensitive in secondary dengue. For people who have previously been infected with dengue there is a particular need to avoid subsequent infections. Public Health laboratories should note that diagnoses of secondary episodes of dengue can be problematic and laboratories should seek expert advice.
Confirmation of the laboratory result by a second arbovirus reference laboratory is required if the case was likely to have been acquired in Australia outside known dengue receptive areas. A list of arbovirus reference laboratories appears in Appendix 1. Virus culture is used for genotyping but is not sensitive enough for routine diagnostic testing.
Antigen detection
Detection of non-structural protein 1 (NS1) can provide a sensitive and specific alternative to PCR and is also useful for dengue diagnosis during the first week of illness (as early as 1 day post-infection) particularly before the development of dengue-specific IgM or IgG. However, as an antigen test it cannot detect past (historical) infections (> 18 days). Due to its specificity, the non-structural antigen (NS1) test can be used to differentiate dengue from other suspected flavivirus infections. NS1 detection is by enzyme immunoassay either in plate assay or on lateral flow immunoassays (rapid antigen tests). The NS1 antigen is usually less labile and thus less susceptible to suboptimal storage and transport conditions. It does not differentiate between dengue serotypes.
Nucleic acid testing
PCR diagnosis for dengue is now common and is performed by many reference laboratories. PCR can provide a rapid result (within a day of receipt) and allows the infecting serotype to be identified. Its sensitivity is high (80-100%) in detecting virus during the acute phase of the disease although the viral RNA may be affected by transport and storage conditions. Therefore specimens of whole blood, serum, plasma and tissues for PCR need to be refrigerated at 4 to 8oC. A ‘detected’ dengue PCR test is confirmation of a recent dengue virus infection. A ‘not detected’ result however, must be interpreted with caution and in conjunction with antigen detection, IgM and IgG results.
Serology
Enzyme linked immunosorbent assay (ELISA) kits for DENV antibodies can provide a rapid result but are not specific for dengue; a positive result could indicate a cross-reaction or a recent flavivirus infection other than dengue. IgG ELISA is used for detection of recent or past dengue infections in acute and convalescent paired sera but is not specific for dengue and should be interpreted carefully within the context of the clinical illness and exposure history.
In Australia, much of the testing of human sera for evidence of DENV infection in state pathology and private diagnostic laboratories involves the use of commercially-available antibody kits. Detection of IgM by enzyme immunoassay (EIA) is usually positive if samples are taken 5 days or more after onset of fever, and it persists for several months. Dengue IgG appears shortly afterwards and persists indefinitely. Whilst these kits measure antibodies to DENV, flavivirus IgG is highly cross-reactive, so the tests will also be positive for other flavivirus infections. IgM is more specific, but still shows substantial cross-reactivity with IgM due to other flavivirus infections.
A significant rise in IgG or seroconversion indicates recent infection, but is not specific for dengue unless confirmed by a specific IgG test such as neutralizing antibody titres. Neutralizing titres can also be used to identify the infecting serotype and to distinguish between primary and secondary dengue. In the latter situation the acute serum will contain antibody to the previously infecting serotype, while the convalescent serum will demonstrate a rise in antibody to the currently infecting serotype. Advice on diagnosis of suspected secondary dengue should be sought from diagnostics experts.
Test results should be interpreted carefully within the context of the clinical illness and exposure history. These kits are useful for diagnosing DENV infection in regions where the virus is endemic or during proven epidemics, when the likelihood of illness being due to dengue is relatively high. In Australia, where DENV is not endemic and prevalence of past infection is much lower, the use of such kits with low specificity increase the risk of false positive results that may be misleading for health authorities. Wherever possible these results should be confirmed by NS1 antigen or PCR positivity.
In-house EIAs have been developed in some Australian laboratories and the interpretation depends on the design and performance characteristics of those assays. Currently there are no EIA assays that can reliably distinguish between dengue antibody and that due to other flaviviruses.
9. Case management
Response times
Investigation should begin on the same day of notification of a confirmed or probable case, in order to determine whether a response is required from the local Public Health Unit to prevent further local transmission. If any case is suspected to be acquired in Australia but diagnosed outside the jurisdiction where it was acquired, the communicable diseases control branch in the state or territory of acquisition should be notified on the same day to ensure case/contact management and mosquito control measures can begin promptly.
NSW specific advice
Investigation following notification should begin on the same day. Exposures, travel history and risk history will determine whether the response is considered urgent or routine. Response is urgent for cases currently in dengue-receptive areas and/or cases who have been in dengue receptive areas whilst viraemic. Routine response is required for cases in areas not receptive to dengue transmission.
Response procedure
Case investigation
Investigate using the dengue investigation form (see example form, Appendix 2).
PHU staff should ensure that action has been taken to:
- confirm the onset date, travel history and symptoms of dengue illness
- confirm results of laboratory tests
- where possible seek the doctor’s consent to contact the case or relevant care-giver
- interview the case or carer and determine source of dengue infection. See Exposure investigation. For locally acquired cases, consult with the relevant jurisdiction to determine who is best placed to interview the case in detail about likely place of acquisition.
Exposure investigation
If possible determine whether the case, during the exposure period (3-14 days prior to onset of symptoms) travelled to regions where DENV is endemic.
If a case has not travelled to a dengue-endemic are during the exposure period (3-14 days prior to onset of symptoms) consider local transmission of dengue and immediately notify NSW CD On Call to arrange for expert investigation and control advice.
One case of locally acquired dengue in a receptive area constitutes an outbreak. If a case who is considered viraemic stays in, or travels to, a receptive area (see
Figure 1 Map; currently only areas within Queensland):
- identify where the case lived/worked/visited while viraemic
- notify CDOnCall who will share case information with Queensland Health officials responsible for communicable disease control.
Case treatment
Case treatment is the responsibility of the attending doctor. No specific treatment for dengue is available.
Education
Cases and other potentially exposed people should be provided with written information on dengue to help them prevent or manage dengue infection. The Queensland Health Mosquito-borne Diseases website has specific resources that can be accessed, including links to the
Queensland Health dengue fact sheet
Isolation and restriction
Infected people notified in NSW should be strongly advised to not travel to regions in Australia where there is the potential for dengue transmission during the period they are likely to be viraemic (while they are symptomatic, usually 3 - 5 days).
Infected people in receptive areas should be advised to avoid being bitten by mosquitoes for up to twelve days from onset of illness to avoid passing dengue virus onto mosquitoes, which can facilitate further transmission to humans.
Advise to use mosquito repellent, wear protective clothing, use window screens or insecticide treated bed nets and plug-in insecticide vaporisers where possible within the home. Spatial repellents designed for indoor use (typically plug-in emanation devices containing synthetic pyrethroids such as d-allethrin) should be encouraged for use in rooms where infected people are resident.
Infected people should not donate blood until four weeks after complete recovery.
Active case finding
Active case finding should occur following identification of a case acquired or present while viraemic in receptive areas. The purpose is to quickly establish whether an outbreak is occurring and direct control efforts to new areas where cases are found. The methods used to actively find more cases include:
- active case finding among residents in the exposure area who are or have been symptomatic and advising any symptomatic residents to see their doctor for appropriate dengue testing
- alerting GPs, laboratories and EDs to the case requesting consideration of dengue in recent and future cases with dengue compatible illness and ensuring appropriate dengue tests are ordered for those cases that may arise
- providing information to institutional settings such as schools, aged care, prison, workplaces in the exposure area, to elicit more cases.
10. Environmental risk management
Urgent determination of the risk of further dengue transmission is important when a case is notified. If the case is not known to have been imported (i.e. there is no travel history for the patient), local transmission should be suspected.
Evaluating the risk for further dengue transmission should be done by a public health clinician in consultation with the medical entomologist and/or local environment health authority and should include assessment of locations a viraemic case has been during the incubation period (to ascertain place of acquisition) and also visited while infectious (to ascertain risk of transmission). The aim of this assessment is to identify areas where transmission is possible, actively search for any further cases and to direct mosquito elimination activities. This includes identifying common breeding sites and elimination of both larval and adult populations of
Ae. aegypti and
Ae. albopictus.
Such investigations should include the recommended vector surveillance and control activities specifically detailed in the
Framework for surveillance and control of dengue virus infection in Australia, and the Queensland Health Dengue Management Plan, available from the Queensland Health website. In brief, these include surveillance of both larval and adult
Ae. aegypti within a 100m radius of a case house, and control of mosquito larvae and adults within a 200 m radius of the home of each case. Special attention should be paid to nearby residences (including tourist accommodation) considered to be high risk for mosquito breeding.
11. Contact management
Contacts of an infected person cannot be infected by person-to-person transmission, but may be infected through a mosquito transferring virus from one person to another. Therefore, this section provides details on the public health response for co-exposed persons.
Contact management is
only required where the disease is acquired or thought to be acquired in an Australian receptive area
or where the disease is acquired overseas and the case spent time in an Australian receptive area while viraemic.
Identification of contacts
Although there is no direct person to person transmission (apart from through blood transfusion), there is a need to follow up persons who have hade the same exposure as the case in receptive areas.
Contact definition
Persons who are epidemiologically linked and may have the same exposure as the case, including household members and persons who work or travelled with the case. This might lead to identification of the index or imported case.
Prophylaxis
Not applicable.
Education
Written information should be made available to people who have been potentially exposed to dengue to advise them of appropriate measures to prevent and/or manage dengue infection.
Isolation and restriction
Not applicable unless symptomatic, then the same precautions apply as case management i.e. avoid being bitten by mosquitoes during the first 5 days of illness.
12.
Special situations
Outbreaks
As dengue is not endemic to Australia, local dengue outbreaks in receptive areas usually begin with a single imported case. A confirmed case of locally acquired dengue anywhere in Australia, or a confirmed or probable case in dengue receptive areas (i.e. case has no history of travel beyond the local area within the exposure period for dengue: 3-14 days prior to onset of symptoms) requires an outbreak response. An interval of approximately two weeks is expected between generations of human cases due to the incubation periods in infected mosquitoes and subsequently infected humans, however, the shortest possible interval is 8 days (see 2. The Disease, Incubation period). In an outbreak, an incident management team including a medical entomologist and local government representatives should be formed and the outbreak managed according to current dengue management plans.7 Once the outbreak is established, the focus is on a community level approach to eliminating mosquitoes.
Some additional issues the PHU should address during an outbreak are:
- informing the wider public health network, local government and the Department of Agriculture of the outbreak to increase surveillance and prepare for outbreak response
- communicating regularly with GPs, hospital emergency departments and local pathology laboratories to ask them to be alert for dengue cases
- maintaining epidemiological information of cases e.g. timeline and mapping of cases
- regular communication with medical entomologists and any charged with vector control responsibilities so that vector control measures can be appropriately directed
- case finding in new dengue areas may become the priority as the outbreak develops
- using media alerts and updates to communicate key messages during the outbreak
- liaising with the Australian Red Cross Blood Service as blood donation services in outbreak locations may be restricted.
13.
References
-
Heymann DL. Control of Communicable Diseases Manual. 19 edn: American Public Health Association; 2008.
-
Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, et al. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 2013;8(7):e68137.
-
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010;8(12 Suppl.):S7-16.
-
World Health Organization. Dengue guidelines for diagnosis, prevention and control. World Health Organization: Geneva, 2009. Available from:
http://www.who.int/topics/dengue/en/index.html.
-
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013;496(7446):504-507.
-
World Health Organization. Dengue and dengue haemorrhagic fever. Fact Sheet No.117. Geneva: World Health Organization; [updated March 2009; cited 20 January 2010]. Available from:
http://www.who.int/mediacentre/factsheets/fs117/en/index.html.
-
Russell RC, Currie BJ, Lindsay MD, Mackenzie JS, Ritchie SA, Whelan PI. Dengue and climate change in Australia: predictions for the future should incorporate knowledge from the past. The Medical Journal Of Australia 2009;190(5):265-268.
-
Queensland Health.
 Queensland Dengue Management Plan 2010-2015 . Published 2011.
Queensland Dengue Management Plan 2010-2015 . Published 2011. -
Fitzsimmons GJ, Wright P, Johansen CA, Whelan PI. Arboviral diseases and malaria in Australia, 2008-09: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. 2010;34(3):225-240.
-
McBride WJH. Deaths associated with dengue haemorrhagic fever: the first in Australia in over a century. Med J Aust 2005;183(1):35-37.
-
Knope K, National Arbovirus and Malaria Advisory Committee, Giele, C.,. Increasing notifications of dengue related to overseas travel, 1991 to 2012. Commun. Dis. Intell. 2013;Vol. 37 (1).
14.
Appendices
15. Jurisdiction specific issues
Links to state and territory public health legislation, the Quarantine Act 1908 and the National Health Security Act 2007