Free chlorine
Free chlorine is measured using the DPD No. 1 tablet. Free chlorine consists of two chemicals; hypochlorous acid and the hypochlorite ion. Hypochlorous acid disinfects well while the hypochlorite ion does not.
Free Chlorine = Hypochlorous acid (HOCl) + Hypochlorite ion (OCl-)
DPD1 (good disinfectant) (poor disinfectant)
Ultra-violet (UV) light degrades hypochlorous acid to hydrochloric acid (HCl). About 35% of the free chlorine can be destroyed every hour of exposure to UV light in outdoor swimming pools. UV light does not penetrate glass, plastic or polycarbonate and therefore does not affect indoor or enclosed swimming pools. UV light does not degrade free bromine. UV light therefore reduces the concentration of the active disinfectant component of free chlorine.
Stabiliser
Stabiliser is the generic name given to the use of cyanuric acid (also known as iso-cyanuric acid) or its chlorinated compounds of sodium dichloro-isocyanurate and trichloro-isocyanuric acid. When added to an outdoor swimming pool cyanuric acid bonds loosely to hypochlorous acid to minimise degradation by UV light. The following graph shows the effect on the loss of free chlorine as the concentration of cyanurate rises.
Graph 1: Cyanuric acid concentration vs percentage free chlorine loss in one hour
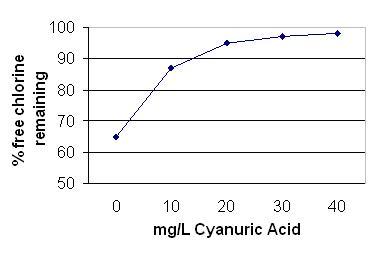
- about 65% of free chlorine remains after 1 hour of strong sunlight on an outdoor swimming pool without cyanuric acid
- at a concentration of 10 mg/L of cyanuric acid the loss of chlorine is reduced to about 12%
- at a concentration of 20 mg/L of cyanuric acid the loss of chlorine is reduced to about 5%
- at a concentration of 30 mg/L of cyanuric acid the loss of chlorine is reduced to about 3%
- a concentration of 40 mg/L of cyanuric acid the loss of chlorine is reduced to about 2%
- further addition cyanuric acid does not produce any substantial benefit in reduced free chlorine destruction. This follows the law of diminishing returns.
While the addition of cyanuric acid reduces the degradation of free chlorine by UV light there is also a distinct disadvantage. Increasing concentrations of cyanuric acid reduces both the disinfection power and oxidation reduction potential (ORP) of free chlorine. To offset the loss of disinfection power of free chlorine in stabilised outdoor pools the minimum concentration of free chlorine must be increased from 1 mg/L to 3 mg/L and the concentration of cyanurate must not exceed 50 mg/L. The optimal concentration of cyanuric acid is 30 mg/L. A nett saving of chlorine chemical will be realised when cyanurate is used in outdoor pools even at the higher free chlorine concentration.
Forms of stabiliser
Stabiliser is available as cyanuric acid, which does not contain chlorine and lowers the pH when added to swimming pool water. Stabiliser is also available as sodium dichloro-isocyanurate (dichlor) which contains between 56% to 62% available chlorine depending on the formulation and does not affect the pH of the pool water when added. Trichloro-isocyanuric acid (trichlor) contains 90% available chlorine that dissolves slowly and is highly acidic when added to pool water.
Use of stabiliser
Cyanurate is not consumed or lost from swimming pool water unless due to splashing or backwashing. If free chlorine, which is continually consumed by disinfection and oxidation, is continually added using dichlor or trichlor then the cyanuric acid concentration will continuously increase and soon exceed the upper limit of 50mg/L. Without discontinuing the use of dichlor or trichlor the cyanuric acid level may increase uncontrolled to levels which will prevent free chlorine from effective disinfection and oxidation.
The proper use of stabiliser is to add cyanuric acid, dichlor or trichlor until the cyanuric acid reaches 50 mg/L. At this concentration a non-cyanurate containing chlorine product such as sodium, calcium or lithium hypochlorite should be used until the cyanuric acid concentration drops to 25 mg/L. Dosing using a cyanurated chlorine product may then be resumed until 50 mg/L is again reached.
Alternatively the pool could be slug dosed with cyanuric acid at night or when no swimmers are in the pool for 8 hours to attain 50 mg/L. The cyanurate concentration should then be allowed to drift down to 25mg/L over the following weeks and then boosted back to 50mg/L. The use of cyanuric acid will lower pH.
A stabilised pool must operate at a minimum of 3 mg/L free chlorine and stabiliser must not be used in an indoor pool or with bromine treated swimming pools. Cyanurate must not be used under any circumstances in a spa pool.
Legislation
Schedule 1 of the Public Health Regulation 2012 controls the levels of cyanurate in public swimming pools and spa pools. In clause II a maximum concentration of 50 mg/L of cyanuric acid is prescribed.
In clause 4(2)(b) a minimum free chlorine concentration of 3mg/L is prescribed in outdoor public pools where cyanurate is used.
Further information
The Public Swimming Pool and Spa Pool Advisory Document provides detailed explanations and information on disinfection, pool chemistry, risk assessment and other issues relevant to swimming pool operation.
Public swimming pool issues may be discussed with an environmental health officer at a local Public Health Unit by calling 1300 066 055, or at your local council.